Article Text
Abstract
Interleukin (IL)−6 inhibition has been approved for the treatment of rheumatoid arthritis, systemic juvenile arthritis, polyarticular juvenile idiopathic arthritis, giant cell arteritis, and, in some countries, Castleman’s disease. IL-6 has also been implicated in several non-rheumatoid arthritis inflammatory and immune conditions such as systemic sclerosis, vasculitides, systemic lupus erythematous, and psoriatic arthritis. In orphan diseases, such as systemic sclerosis, which are associated with significant morbidity and mortality and for which there are no approved treatments, IL-6 inhibition may offer a promising treatment strategy. It is also becoming clear that IL-6 may have an important role not only in inflammatory and immune diseases but also in non-immune mediated diseases such as endogenous depression and depression associated with chronic inflammatory conditions. Several studies have explored the effect of IL-6 pathway inhibition in Crohn’s disease and adult-onset Still’s disease, suggesting that IL-6 may be important in their pathogenesis.
Statistics from Altmetric.com
A panel of international experts in the field of rheumatology recently came together to consider indications beyond rheumatoid arthritis for IL-6 pathway inhibitors.
Considering the role of IL-6 in non-rheumatoid arthritis inflammatory and immune disease
IL−6 inhibition is effective and approved for the treatment of several inflammatory diseases including rheumatoid arthritis, systemic juvenile arthritis, polyarticular juvenile idiopathic arthritis, and giant cell arteritis. In some countries, it is also approved for the lymphoproliferative disorder Castleman’s disease. However, IL-6 inhibition is ineffective in the treatment of certain other inflammatory diseases such as ankylosing spondylitis. For example, the BUILDER one study, which compared the human anti-IL-6 receptor (IL-6R) monoclonal antibody tocilizumab with placebo in patients with ankylosing spondylitis,1 tocilizumab was ineffective in treating tumour necrosis factor (TNF) inhibitor-naive patients. In the accompanying sections, we will review the evidence that supports the application of IL-6 blocking interventions in the management of specific diseases.
Systemic sclerosis
Systemic sclerosis is an autoimmune disease causing significant morbidity and mortality. There is no approved treatment for this disease. Evidence shows that polymorphisms in the IL-6 gene are associated with systemic sclerosis. In patients with systemic sclerosis, spontaneous production of IL-6 and soluble IL-6R (sIL-6R) by peripheral blood leukocytes is elevated compared with healthy controls. This increase in IL-6 concentrations is correlated with the modified Rodnan skin score, with patients with high IL-6 concentrations tending to have worse outcomes.2
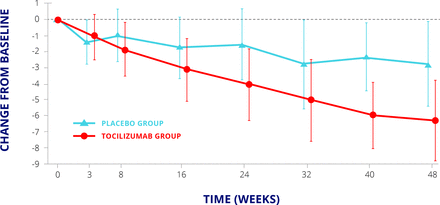
The FaSScinate study3 was a small phase II randomised proof of concept study, which randomised 87 patients with systemic sclerosis to either placebo or subcutaneous tocilizumab treatment. Many of the patients had chronic established systemic sclerosis. The primary endpoint was the difference in mean change from baseline in modified Rodnan skin score at week 24. The least squares mean change in modified Rodnan score at 24 weeks was –3.92 in the tocilizumab group and –1.22 in the placebo group (difference –2.70, 95% CI –5.85 to 0.45; P=0.0915). At the end of the study, week 48, there was numerical difference with less progression in skin thickening observed in the tocilizumab group (–6.33) compared with the placebo-treated group (–2.77) (figure 1). The difference just failed to reach statistical significance (treatment difference –3.55,–7.23 to 0.12; P=0.0579), which may be a result of the small number of patients in each group.
FaSScinate trial: modified Rodnan skin score.3
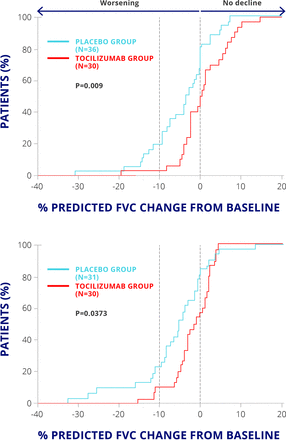
Importantly, patients who were treated with tocilizumab had less reduction in forced vital capacity at 48 weeks than the placebo-treated group (P=0.0373) (figure 2). Therefore, IL-6 inhibition may be a promising strategy for this orphan disease for which there is currently no effective treatment.
FaSScinate trial: forced vital capacity.3
Vasculitis syndromes
Other conditions in which IL-6 has been implicated include the vasculitides. The association between cytokines and different vasculitides are listed in table 1.4–7 Elevated IL-6 activity is often associated with active disease in many vasculitides (table 1).
Vasculitis syndromes: associated cytokines4–7
In patients with Takayasu’s arteritis, a disease affecting the large blood vessels, serum IL-6 concentrations have been shown to be elevated significantly during the active phase of the disease compared with healthy controls.7 In Behçet’s disease, although increased concentrations of IL-6 are seen during active disease and remission, they are higher during active disease.8
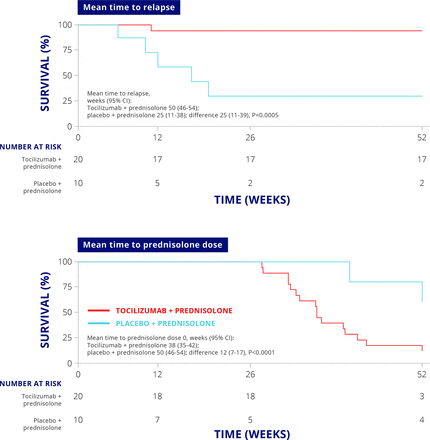
A phase II randomised, placebo-controlled trial of tocilizumab in giant cell arteritis recruited patients with giant cell arteritis who were aged 50 and over and had new onset or relapsing disease.9 Thirty patients were randomised 2:1 to receive either tocilizumab (8 mg/kg) or placebo intravenously. Thirteen infusions were given at 4-week intervals until week 52. Both groups received oral prednisolone, starting at 1 mg/kg per day and tapered down to zero according to a standard reduction scheme defined in the study protocol. The primary outcome was the proportion of patients who achieved complete disease remission at a prednisolone dose of 0.1 mg/kg/day at week 12. The mean time to relapse and the mean time to prednisolone dose were longer in the tocilizumab group than in the placebo treated group (figure 3). These differences were statistically significant, showing that tocilizumab allows for more rapid withdrawal or tapering of steroid treatment.
Clinical efficacy of tocilizumab in giant cell arteritis.
The placebo-treated group had a higher incidence of cardiovascular complications (five patients) than the tocilizumab-treated group (one patient).9
The GiACTA study, a 52-week, phase III, global, randomised, double-blind, placebo-controlled trial investigating the efficacy and safety of tocilizumab in patients with giant cell arteritis, has also shown the efficacy of adding tocilizumab to steroid tapered therapy in patients with giant cell arteritis. Tocilizumab treatment combined with prednisone tapering over 26 weeks was superior to placebo plus prednisone tapering over 26 or 52 weeks in terms of patients achieving sustained glucocorticoid remission.10 Recently, the FDA approved tocilizumab for the treatment of giant cell arteritis in the US.11
There is also evidence for involvement of IL-6 in polymyalgia rheumatica, with patients having increased serum IL-6 concentrations compared with normal controls, and changes in serum IL-6 concentrations correlating with clinical manifestations during prolonged corticosteroid therapy.12 Because polymyalgia rheumatica and giant cell arteritis are clinically related syndromes, by extrapolation there is a strong interest in the potential of using tocilizumab therapy in patients who have long-standing polymyalgia rheumatica requiring high doses of steroids.
Systemic lupus erythematosus
Systemic lupus erythematosus (SLE) is an autoimmune disease characterised by autoantibody formation resulting in diverse symptoms. Patients with SLE have elevated serum concentrations of IL-6, which are higher in active versus inactive disease.13–15 Serum IL-6 was shown to correlate with SLE activity index (SLEDAI), erythrocyte sedimentation rate (ESR), and serum C reactive protein (CRP) concentrations13 and to correlate with clinical manifestations such as anaemia.16 In a murine model of SLE, treatment with an anti-murine IL-6 monoclonal antibody showed beneficial effects on autoimmunity and suppressed the production of anti-double-stranded deoxyribonucleic acid (anti-dsDNA).17 In this context, anti-IL-6 monoclonal antibody inhibited B cell and T cell proliferation and mixed lymphocyte reactions and prevented the development of severe kidney disease.17
In a small open-label study, 16 patients with mild to moderate SLE were treated bi-weekly for 12 weeks with one of three doses of tocilizumab: 2, 4, or 8 mg/kg.18 Patients were followed for an additional 8 weeks. Tocilizumab reduced disease activity in patients with SLE, and significant improvements in SLEDAI and Systemic Lupus Activity Measure (SLAM) scores were observed after 14 weeks. Arthritis improved in all seven patients with symptoms at baseline, and four patients saw their symptoms resolved. Illei et al also showed that tocilizumab therapy reduces autoantibody production in SLE: the frequency of plasma cells decreased significantly over 14 weeks; levels of anti-dsDNA autoantibodies decreased by a median of 47% in patients treated with 4 mg/kg and 8 mg/kg doses of tocilizumab.
In a recent dose-ranging randomised controlled trial, Wallace et al evaluated PF-04236921, a fully human immunoglobulin G2 monoclonal antibody that binds and neutralises IL-6, in patients with active SLE at doses of 10 mg, 50 mg, or 200 mg over a 24-week double-blind treatment phase.19 While there was no significant difference from placebo among the treatment arms for the primary endpoint (proportion of patients achieving the SLE Responder Index (SRI-4) at week 24, there was improvement measured in the primary and key secondary end points with the 10 mg dose. Additionally, in a post-hoc analysis, the subgroup of patients with high disease activity at baseline showed higher response rates with the 10 mg dose. To date, there has been no large placebo-controlled trial in this condition.
Psoriatic arthritis
Evidence exists that IL-6 concentration is elevated in patients with psoriatic arthritis, with patients having higher serum and synovial concentrations of IL-6 compared with healthy volunteers and patients with skin psoriasis, though lower levels than rheumatoid arthritis patients.20–23 Elevated IL-6 in patients with psoriatic arthritis has also been shown to correlate with the number of painful and swollen joints, rheumatology attitudes index, physician’s assessment of disease, serum CRP, and ESR.20 22
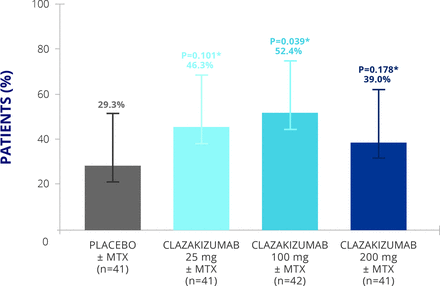
In a 24-week randomised, double-blind, placebo-controlled, dose-ranging study, patients with active psoriatic arthritis were randomised 1:1:1:1 to receive subcutaneous placebo or subcutaneous clazakizumab, an anti-IL-6 monoclonal antibody, at dosages of 25, 100, or 200 mg every 4 weeks with or without methotrexate.24 The primary endpoint was response rate according to ACR20 at week 16. This study showed that clazakizumab therapy was able to significantly improve arthritis score, with approaching 50% of patients achieving ACR20 response in the clazakizumab-treated group compared with 29% receiving placebo treatment (figure 4).
Clinical efficacy of clazakizumab in psoriatic arthritis.24 MTX, methotrexate.
However, only small improvements in skin disease were reported (table 2). This study shows that clazakizumab could be useful in improving physical function in patients with psoriatic arthritis who have well-controlled skin disease.
Clazakizumab PASI scores in psoriatic arthritis
Considering the role of il-6 in non-immune mediated disease
Depression
A more controversial topic is the role of IL-6 in non-immune mediated conditions such as endogenous depression. The ability of IL-6 to mediate function of different tissues is well known, and IL-6 and the soluble receptor system are well designed to modify systemic effects in tissues and organs away from the primary site of inflammation or disease activity.25–27 The prediction, therefore, is that if we interfere with IL-6 biology we will likely see consequential changes in metabolic and psychological neuroplasticity systems. Available data suggest that some degree of balance needs to be maintained between pro-inflammatory and anti-inflammatory cytokines to regulate synaptic plasticity and memory formation in individual neurons and the whole brain.28
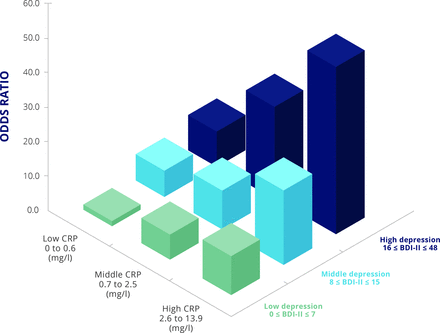
It is well known that depression is common in patients with chronic inflammatory conditions. A major survey in patients with arthritis found that one third of respondents had at least one of anxiety and/or depression.29 There is also evidence that inflammatory conditions are strongly associated with depression.30 31 Figure 5 illustrates that patients with rheumatoid arthritis are more likely to have depression, especially if they have active arthritis, as reflected by increased CRP concentration, with CRP concentration associated with the severity of depression.30
Increased CRP concentration is associated with depression in rheumatoid arthritis.30 BDI-II, Beck Depression Inventory II score; CRP, C-reactive protein.
It has long been supposed that the reason why chronic inflammatory disease causes depression is that cytokines such as IL-6 and TNFα can reach the brain through the hypothalamic-pituitary-adrenal axis.32 In addition, it is also proposed that these cytokines act on the blood-brain barrier to relay inflammatory signals from the periphery to the brain.33 In hepatitis C, an inflammatory condition of the liver, type-1 interferon treatment may yield depressive symptoms by lowering brain serotonin levels, altering IL-6 and IL-8 concentrations and increasing cortisol and adrenocorticotropic hormone concentrations.34 The findings from a systematic review and meta-analysis of clinical trials of chronic inflammatory conditions suggest that cytokines may have a causal role in depression, with the implication that cytokine modulators may be novel drugs for depression in patients with chronic inflammation.35
The role of cytokines in endogenous depression is controversial. However, a possible role of cytokines, including IL-6, in endogenous depression, as well as depression that occurs in patients with chronic inflammatory disease, is biologically plausible. A meta-analysis has provided strong evidence that concentrations of cytokines, including TNFα and IL-6, are elevated in patients with major depression.36
The findings above have led to interest in IL-6 inhibition as a possible treatment for depression and associated disorders.37 Improvement in mental health and mental component scores in anti-IL-6 therapy (sirukumab) have been observed in the SIRROUND-T trial, a randomised, double-blind, placebo-controlled, phase III study, which examined efficacy and safety of sirukumab in patients with active rheumatoid arthritis refractory to anti-TNF therapy.38 Sirukumab 50 mg every 4 weeks or 100 mg every 2 weeks showed improvement in mental health component score compared with a placebo-treated group. Therefore, in patients with rheumatoid arthritis who have associated depression, it seems that IL-6 inhibition may be able to improve the mental health. The question remains as to whether this may be unique to IL-6 blockade, and whether it is a specific effect of IL-6 cytokine blockade as opposed to the receptor blockade, as this was not seen in the RADIATE study of tocilizumab in patients with rheumatoid arthritis and inadequate responses to TNF inhibitors.39 A randomised, controlled trial is under way to examine the effect and safety of sirukumab in major depressive disorder. However, following a negative review from the FDA, sirukimab development and registration has been stopped.
There is a need to train physicians to be more comfortable in talking to patients about mood. We recognise that one of the real challenges for people with mental health problems in most developed societies is the stigma associated with acknowledging that symptoms exist. Yet, in addition to a reaction to a set of circumstances, there is real biology underlying depression.
Considering IL-6 to immunity and beyond—the future
Various off-label uses of tocilizumab are being reported in the literature. A pilot randomised trial has been conducted in active Crohn’s disease, suggesting a clinical effect40; a case series has been reported in adult-onset Still’s disease41 and a case report in Takayasu’s arteritis,42 indicating that IL-6R inhibition with tocilizumab may be a future treatment option for these conditions. A study has also shown that tocilizumab may have activity in the corticosteroid refractory graft versus host disease and in chronic kidney rejection.43
Individual case reports also exist in amyloidosis,44 polymyositis,45 and refractory relapsing polychondritis.46 There have also been reports of successful treatment of refractory diseases, including Behçet’s disease, uveitis, and TNF receptor-associated periodic syndrome.47–49
Heterogeneity in synovial phenotypes may explain heterogeneity in response to drug therapy in rheumatoid arthritis and possibly other autoimmune diseases. Identifying and stratifying patients by synovial phenotype, using serum biomarkers, may assist in future clinical decision making.50
IL-6 pathway inhibition may provide hope as a treatment strategy, not only for rheumatic diseases with no currently approved treatment options, but also for other conditions such as giant cell arteritis, with potential as a glucocorticoid sparing approach to treatment. Targeting IL-6 may also represent a possible future treatment or disease modification approach for patients with depression with or without a chronic inflammatory condition.
References
Footnotes
Funding This initiative is sponsored by R-Pharm through the provision of an unrestricted educational grant. R-Pharm has had no influence over the content.
Competing interests DA declares no conflicts. EHC reports grants from NovImmune AG, grants and personal fees from Pfizer, grants from UCB, grants and personal fees from Roche, personal fees from Abbvie, Biogen, Bristol Myer Scripps, Chugai Pharma, Eli Lilly, Hospira, Janssen, Novartis, Regeneron, R-Pharm and Sanofi-Aventis; SAJ reports non-financial support from CESAS Medical during the conduct of the study; personal fees from CESAS Medical, Eleven Biotherapeutics, grants and personal fees from Roche Pharmaceuticals, personal fees and other from Genentech, grants and personal fees from Glaxo-Smith-Kline, Chugai Pharmaceuticals, Ferrin Pharmaceuticals, Regeneron/Sanofi, grants, personal fees and non-financial support from NovImmune AG, outside the submitted work; IM reports grants from Roche and Refereron, during the conduct of the study; grants and personal fees from Abbvie, Astra Zeneca, Celgene, GSK, Janssen, Lilly, Novartis, Pfizer, Roche, grants and personal fees from Refereron, outside the submitted work; JS reports grants from Abbvie, Astra-Zeneca, Janssen, Lilly, MSD, Pfizer, Roche, personal fees from Abbvie, Amgen, Astra-Zeneca, Astro, BMS, Celgene, Celltrion, Chugai, Gilead, Glaxo, ILTOO, Janssen, Lilly, Medimmune, MSD, Novartis-Sandoz, Pfizer, Roche, Samsung, Sanofi, UCB during the conduct of the study; TT reports grants, personal fees and other from Astellas Pharma Inc, Abbvie GK, Mitsubishi Tanabe Pharma Co, grants and personal fees from Bristol–Myers KK, Chugai Pharmaceutical Co Ltd, Daiichi Sankyo Co, Ltd, Pfizer Japan Inc, grants from Takeda Pharmaceutical Co, Ltd, Teijin Pharma Ltd, Asahikasei Pharma Corp, Eisai Co Ltd, AYUMI Pharmaceutical Corporation, grants and other from Taisho Toyama Pharmaceutical Co Ltd, Nipponkayaku Co Ltd, other from Astra Zeneca KK., Eli Lilly Japan KK, Novartis Pharma KK, Janssen Pharmaceutical KK, outside the submitted work; The participants/authors received personal fees for their participation in the round table.
Patient consent Not required.
Provenance and peer review Commissioned; externally peer reviewed.